Dr. Mark E. Peterson talks feline hyperthyroidism during an interview with Steve Dale, the host of Steve Dale's Pet World. You may click through to subscribe to the Animal Endocrine YouTube Channel to keep on top of new video uploads. Dr. Peterson and the Animal Endocrine clinic can also be followed on our Animal Endocrine blog, our blog for vets, our website, on Facebook and on Twitter.
Źródło: animalendocrine.blogspot.com
Cushing?s syndrome, also called hyperadrenocorticism, is a disorder which results from the excess production of an adrenal hormone called cortisol. It is a common endocrine disease in middle-aged and older dogs. Miniature Poodles, Dachshunds, Boxers, Boston Terriers, and Beagles are particularly vulnerable.
What causes Cushing's syndrome in dogs?
There are three major causes for Cushing?s syndrome. The most common cause (85?90% of cases) is a small tumor in the pituitary gland. The pituitary tumor produces a hormone (adrenocorticotropic hormone or ACTH) that causes the adrenal gland to grow and oversecrete cortisol.
Less commonly (10?15% of cases), the adrenal glands themselves develop a tumor that secrete cortisol.
A third cause of Cushing's syndrome in dogs results from the long-term use of high doses of corticosteroid drugs such as prednisone or dexamethasone. These steroid drugs are used to decrease inflammation or treat an immune disorder.


What clinical signs do dogs with Cushing's syndrome develop?
Common clinical signs include the following:
* increased thirst and urination
* increased appetite
* excessive panting
* lethargy
* pot belly appearance
* weight gain
* hair loss
How do we diagnose Cushing's disease in dogs?
Diagnosing Cushing?s syndrome can be difficult. Laboratory test results may be inconclusive and dogs suffering from other diseases commonly show false-positive test results for Cushing?s syndrome. (For more information, see this post on my Vet Blog.)
Once we have diagnosed Cushing?s syndrome, the next step is to determine whether the disease stems from a tumor of the pituitary or of the adrenal. This can be done by further endocrine testing or by imaging techniques such as abdominal ultrasound.
How do we treat this problem?
Most dogs with hyperadrenocorticism can be treated with drugs such as mitotane (Lysodren? or trilostane (Vetoryl?). However, these drugs are most safe and effective when used under the supervision of a veterinarian with much experience their use.
If the dog has a tumor of the adrenal gland, surgical removal is generally the best option. Finally, external radiation therapy can help dogs with pituitary tumors, especially large ones.


Źródło: animalendocrine.blogspot.com
I recently read one of your posts on your Animal Endocrine blog site regarding 'How Do We Treat Dogs with Hypothyroidism.' In that post, you indicated that thyroid replacement medication should be given on an empty stomach. And you indicated that this was quite important. My question about this is: I have a number of dogs that are on L-thyroxine but the owners have always given the medication at the time of feeding. If we are doing post-pill serum T4 testing at 4 to 6 hours after administration of the morning dose and adjusting the daily dose according to those thyroid results, does it matter that the owners give the pills with food?
Thank you.
My Response:
Good question. Yes, the absorption of L-thyroxine (L-T4) in dogs has indeed been shown to be much higher when given on an empty stomach.
To my knowledge, this difference in the absorption has only been reported for Leventa, a liquid L-T4 medication (1). In one study, food intake concomitant with L-T4 oral administration delayed L-T4 absorption and decreased its rate and extent by about 45% (2).
I know of no other reports of the effects of feeding on absorption of L-T4 tablets in dogs, but I assume that the differences in absorption might also be true for other brands and formulations of L-T4.
The bottom line: In your patients, giving the L-T4 with food is not wrong. If the post-pill serum T4 is within the therapeutic range and the dog has responded with resolution of the clinical signs of hypothyroidism, I certainly wouldn't change the dose or feeding regime.
My point in that my L-T4 blog post is that if the owners did administer the L-T4 pill on an empty stomach, the daily required dose of L-T4 could likely be lowered. In some dogs, that could be a huge issue in the success of therapy, but in most dogs it probably doesn't make that much difference clinically.
References
1. Leventa (levothyroxine sodium) oral solution. Intervet/Schering-Plough Animal Health. Package insert available at: http://www.leventa.com/default.asp
2. Le Traon G, Burgaud S, Horspool LJ. Pharmacokinetics of total thyroxine in dogs after administration of an oral solution of levothyroxine sodium. J Vet Pharmacol Ther. 2008;31(2):95-101.
Źródło: endocrinevet.blogspot.com
The pituitary hormone corticotropin (adrenocorticotropic hormone or ACTH) is a single-chain 39-amino-acid peptide hormone synthesized in the corticotrophs of the anterior lobe (pars distalis) of the pituitary gland). Although the amino acid sequence of ACTH varies among species, the first 24 amino acids are identical among all species studied to date. Canine ACTH differs from human ACTH by only one amino acid residue, at position 37, although the amino terminal end of the ACTH molecule (amino acids 1 to 18) is responsible for its biologic activity.
ACTH is available in two general forms as a diagnostic testing agent. Both of these forms or preparations of ACTH work by stimulating the adrenal cortex to secrete cortisol, corticosterone, aldosterone, and a few other weakly androgenic substances.
ACTH Gel Preparations
In the past, the main ACTH preparation used for adrenal function testing was ACTH gel, which is extracted from bovine and porcine pituitary glands. The ACTH in these gel preparations is composed of the entire 39-amino-acid sequence of the ACTH peptide.
Brand name ACTH gel preparations
In the USA, the only FDA-approved, brand-name ACTH gel preparation is H.P. Acthar gel Repository Injection (80 U/ml; Questcor Pharmaceuticals). The FDA has specifically labeled H.P. Acthar Gel for use in diagnostic testing of adrenal function. However, the package insert lists a variety of other diseases and disorders for which it may be used including acute multiple sclerosis, infantile spasm, rheumatoid arthritis, hemolytic anemia, allergic conjunctivitis, and ulcerative colitis (1).
 In late 2007, Questcor announced a new strategy and business model for H.P. Acthar Gel Repository Injection (2). The cost of the product was increased from an average wholesale price of $2,063 per 5-ml vial to an estimated $23,000 per vial! The company explained that its price increase was crucial in order to continue manufacturing and distributing this agent to patients who needed it, as well as to fund projects that could contribute to the company’s growth.
In late 2007, Questcor announced a new strategy and business model for H.P. Acthar Gel Repository Injection (2). The cost of the product was increased from an average wholesale price of $2,063 per 5-ml vial to an estimated $23,000 per vial! The company explained that its price increase was crucial in order to continue manufacturing and distributing this agent to patients who needed it, as well as to fund projects that could contribute to the company’s growth.
This increase in price has led physicians to totally abandon its use for adrenal function testing, since synthetic ACTH is so much cheaper. H.P Acthar Gel is almost 29 times more expensive than either formulation of cosyntropin available in the USA (see below). Many have also questioned the therapeutic value of H.P. Acthar Gel, especially as it compares with lower-priced and potentially therapeutically equivalent alternatives, such corticosteroids.
During the late 1970’s and early 80’s, I used this H.P. Acthar gel product routinely for ACTH stimulation testing because it was very cost effective. As the price of Acthar gel started to increase and synthetic ACTH preparations became more available, however, I had completely switched to use of only synthetic ACTH preparations by the late 1980s for adrenal gland testing in dogs and cats.
Today, performing an ACTH stimulation test with H.P. Acthar gel would cost the veterinarian over $1000 per dog just for the product alone, not including the serum cortisol determinations! Obviously, it is highly unlikely that you will be using this ACTH gel preparation for use in testing dogs and cats anytime in the future.
Generic ACTH gel preparations
Generic preparations of ACTH gels (usually 40 U/ml) can be purchased from several veterinary-compounding pharmacies. The following is a partial list of compounding pharmacies that sell such generic ACTH gels: Wedgewood Pharmacy, Pet Health Pharmacy, Red Oak Drug, Meds for Vets, and Diamond Back Drugs. Many practicing veterinarians favor these compounded ACTH products because they are slightly cheaper than Cortrosyn (and certainly much less expensive than H.P. Acthar gel!)
But as someone smart once said, ”you generally get what you pay for.”
There are 4 reasons why I do NOT recommend these compounded ACTH products:
1. Compounding pharmacies are not governed or regulated by the FDA. Therefore, we have no guarantee that the potency of these compounded formulations are what the pharmacy claims them to be. Some batches of compounded ACTH gel may be very potent and maximally stimulate cortisol secretion, whereas others batches or preparations fail to stimulate maximal cortisol secretion or may not stimulate it at all!
2. Because of the potential for lot-to-lot variability in compounded ACTH formulations, one should consider assessing the activity of each new vial by performing an ACTH stimulation test on a normal dog to ensure that the preparation is bioactive (i.e., it will work to stimulate cortisol secretion from the adrenal cortex. Of course, that suggestion is totally impractical for the practicing veterinarians.
3. Because of the differences in potency and absorption of these compounded products, peak ACTH-stimulated cortisol values may occur from 30 minutes to 2 hours after gel administration (3). In contrast to H.P. Acthar gel, where peak cortisol secretion occurred 2 hours after administration, the compounded ACTH preparations are not consistent. Because of this variability in the duration of cortisol response, most authorities recommend collecting post-ACTH at both 1 and 2 hours when using a compounded gel preparation (3).
4. The added time and need to collect and measure a third cortisol concentration offsets any cost savings gained from using a compounded ACTH product. (And remember my second point — we should validate the test with every new vial by testing a clinically normal dog!).
The bottom line: while these compounded ACTH preparations may be less expensive than other available products, they are not recommended because the potency can vary from bottle to bottle.
Stick with synthetic ACTH preparations, as described below!
Synthetic ACTH Preparations
Cosyntropin is a synthetic form of ACTH, created by isolating the first 24 amino acids from the 39-amino-acid ACTH peptide. The only indication for use of cosyntropin is in diagnostic testing of adrenal function. A dose of cosyntropin 0.25 mg, which is biologically similar to a dose of 25 units of ACTH gel, maximally stimulates the adrenal cortex.
Cosyntropin has many advantages over the use of the ACTH gels for ACTH stimulation testing. First of all, the cosyntropin test takes half the time of the ACTH gel test (1 hour vs. 2 hours). Secondly, the ACTH-stimulated cortisol response to cosyntropin is more consistent and variations in potency are not an issue. Finally, cosyntropin is less immunogenic than ACTH gel. The amino acids 22 to 39 in ACTH produce most of the molecule’s antigenicity; thus, cleaving of most of these amino acids from cosyntropin molecule renders it less likely to elicit an allergic response.
In the USA, cosyntropin is either as the brand-name product Cortrosyn (Amphastar Pharmaceuticals) or a generic cosyntropin preparation (Sandoz).
In most countries outside the USA, cosyntropin is called tetracosactide (Synacthen). Despite these differences in name, the chemical structure of tetracosactide is identical to cosyntropin (both 1-24 ACTH).
Cortrosyn
 The brand-name product Cortrosyn is supplied by the manufacturer as a lyophilized powder in vials containing 0.25 mg (250 µg) of ACTH. The cosyntropin powder must be reconstituted with sterile saline solution at time of injection (4).
The brand-name product Cortrosyn is supplied by the manufacturer as a lyophilized powder in vials containing 0.25 mg (250 µg) of ACTH. The cosyntropin powder must be reconstituted with sterile saline solution at time of injection (4).
Unlike ACTH gels, which can be given intramuscularly (IM) or subcutaneously (SQ). Cortrosyn should NOT be administered via the SQ route. However, Cortrosyn be safely given either IM or IV (4).
In dogs, it has been shown that that either IM or IV routes of administration provide equivalent serum cortisol responses. In cats, however, a more consistent and greater adrenocortical response is elicited after IV administration of cosyntropin, so IM administration is not recommended in this species.
Nowadays, it is common practice to dilute Cortrosyn and freeze the diluted aliquots for up to 6 months. This not only extends its shelf life, but makes the use of Cortrosyn much more cost effective.
Generic cosyntropin
Sandoz’s generic cosyntropin, in contrast, is supplied as a solution for injection (0.25 mg per vial).
According to the product insert (5), the synthetic ACTH preparation should be given only intravenously. The product insert also states that cosyntropin injection is intended as a single dose injection and contains no antimicrobial preservative; any unused portion should be discarded.
 To my knowledge, no research studies of this cosyntropin generic preparation have been reported in either the dog or cat. It’s safe to assume that this product would be as effective as Cortrosyn in stimulating adrenocortical secretion. However, it is not know if the IM route would be reliable.
To my knowledge, no research studies of this cosyntropin generic preparation have been reported in either the dog or cat. It’s safe to assume that this product would be as effective as Cortrosyn in stimulating adrenocortical secretion. However, it is not know if the IM route would be reliable.
Even more importantly, we do not know if this preparation can be diluted and stored in the refrigerator or freezer without loss of potency. Until those studies are reported, I would stick with the use of Cortrosyn.
Synacthen
Outside of the U.S., the synthetic ACTH preparation tetracosactide (Synacthen, Novartis) is supplied as a solution for injection (0.25 mg per vial). This preparation appears to be very similar to the generic cosyntropin solution made by Sandoz.
According to my expert sources in the UK and Europe, Synacthen is ‘dirt-cheap.’ So no one has studied if this synthetic ACTH preparation is stable when diluted or how long it’s potency is maintained when stored long-term.
Generally, either one-half or the entire contents of the Synacthen vial are administered per test, depending on the size of the animal.

References
1. H.P. Acthar Gel, Repository Corticotropin Injection, package insert. Union City, CA: Questcor. Available at: http://www.acthar.com/Pdf/Acthar_PI_pdf
2. Questcor Board approves new strategy and business model for H.P. Acthar Gel. Union City, CA: Questcor; August 2007. Available at: http://phx.corporate-ir.net/phoenix.zhtml?c=89528&p=irol-newsArticle&ID=1044912&highlight
3. Kemppainen RJ, Behrend EN, Busch KA. Use of compounded adrenocorticotropic hormone (ACTH) for adrenal function testing in dogs. J Am Anim Hosp Assoc 2005;41:368-372. Abstract available at: http://www.ncbi.nlm.nih.gov/pubmed/16267060
4. Cortrosyn Injection, package insert. Rancho Cucamonga, CA: Amphastar. Available at: http://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?id=3164&type=display
5. Cosyntropin Injection (Generic), package insert. Princeton, NJ: Sandoz. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/022028lbl.pdf
Źródło: endocrinevet.blogspot.com
I have a general question regarding how veterinarians administer and dose the radioiodine ( I-131) as treatment for hyperthyroid cats. Although I have had a number of my hyperthyroid patients treated successfully with radioiodine, I do not know how one administers and doses it. A little explanation would be appreciated.
My Response:
We've been giving the radioiodine subcutaneously since 1986. Prior to that, we initially tried the oral route, but that meant handling radioactive capsules (and hoping the cat's wouldn't chew them) or stomach tubing the cats (and hoping the cats wouldn't vomit). In human patients, they generally put the radioiodine solution into a juice drink to cover up the 'iodine" taste and the people just drink the solution. Obviously, that wouldn't work in cats.
 From around 1980 to 1986 we gave all of the doses IV, which worked fine. However, that meant that two people always needed to be exposed when the dose is administered and the cat needed to have a catheter placed for the injection. The IV administration worked well, but occasionally, I saw anaphylactoid reactions (rather terrifying!) upon treatment. Obviously, there is something in the solution that that cats don't like when the drug is given more than once intravenously.
From around 1980 to 1986 we gave all of the doses IV, which worked fine. However, that meant that two people always needed to be exposed when the dose is administered and the cat needed to have a catheter placed for the injection. The IV administration worked well, but occasionally, I saw anaphylactoid reactions (rather terrifying!) upon treatment. Obviously, there is something in the solution that that cats don't like when the drug is given more than once intravenously.
Today I still administered the radioiodine to all of my hyperthyroid cats by the subcutaneous route. In many of these cats, I can perform the treatment by myself, thereby not exposing another member of my staff to the full dose of radiation that is contained in the syringe. I have NEVER seen an anaphylactoid reaction when the radioiodine solution is given subcutaneously.
As far as dosing goes, I really do believe that facilities that use a 'fixed dose' are overdosing most of the cats, and under-dosing others.
I give a range of doses from 2-10 mCi to cats with benign adenoma (adenomatous hyperplasia). This method of dose determination is somewhat more complicated, and is based on the following factors:
* Clinical severity of hyperthyroidism
* Size of thyroid tumor(s) on palpation
* Result of thyroid scintigraphy (thyroid scanning)
* Age of the cat
* Known concurrent diseases
Cats with thyroid carcinoma generally require much larger radioiodine doses, generally in amounts of around 30 mCi but sometimes even more. These cats generally have larger thyroid masses, generally invading soft tissue and extending into the thoracic cavity.

Hyperthyroid cat with thyroid carcinoma (on left) demonstrating the massive, multinodular tumor, invading and extending into the thoracic cavity. The horizontal line is the region of the thoracic inlet.
My goal of therapy is to 'cure' the hyperthyroid state without causing hypothyroidism. Many treatment facilities boast about the fact that they can cure 98% of hyperthyroid cats. Well, that's easy; anyone can order a big dose for all hyperthyroid cats and cure them, but many will become hypothyroid. I agree it's more difficult to titrate the doses because one has to think about the whole cat and what we are doing, but I do believe it's so very important. That's where this whole treatment issue become more tricky.
It's becoming increasing clear that both hyperthyroidism and hypothyroidism are bad for the kidneys, so that last thing we want to do is cure the hyperthyroidism but create iatrogenic hypothyroidism. And that is especially true if the owners cannot give oral medication or if the cat already has mild renal disease.
Źródło: endocrinevet.blogspot.com
Our pharmacist is telling me that determir insulin (Levemir) expires after 30 days. He claims that this is because of the risk of contamination.
At $112 a bottle, this short expiration date would make detemir cost prohibitive for my clients. Is detemir insulin actually stable for longer periods if kept refrigerated, as has been found with glargine insulin (Lantus)?
My Response:

Glargine is marketed for human use with a 28-day shelf-life at room temperature after opening. Similarly, detemir is marketed with a 6-week shelf-life at room temperature after opening of the vial.
The recommended shelf-lives for both detemir and glargine are relatively short, not because of lack of efficacy, but because of the increased risk of bacterial contamination with these multiple-use,
injectable medication vials. The FDA believes that the insulin vials may have a high probability of becoming contaminated with microbes by the daily multiple punctures needed to withdraw medication when used past the insulin's expiration date.
For veterinary use, we generally recommend that both insulins be kept refrigerated, although theantimicrobial preservative in these insulins may actually be more effective at room temperature. Owners of diabetic cat or dogs use refrigerated glargine or detemir routinely for up to 6 months without evidence of problems occurring. The insulin should be discarded immediately, however, if any cloudiness or discoloration is noted.
This issue with bacterial contamination seems to be extremely rare. Pet owners are much more likely to accidentally drop and break the vial, than to have to throw it away because it develops discoloration.
Źródło: endocrinevet.blogspot.com
Her serum parathyroid hormone (PTH) concentration is high (32.3, normal 3.0-17.0), ionized calcium is high (1.92, normal 1.25-1.45), and the parathyroid hormone-related polypeptide (PTHrp) is negative (0.0, normal 0.0-1.0). No gross evidence of perianal masses are seen including on rectal exam, and no lymph node enlargement can be palpated.
Will this be enough evidence to say that this dog definitely has primary hyperparathyroidism? Or should we do more to rule out other malignancy as the cause of hypercalcemia in this dog?
My Response:
Yes, it certainly does appear that your dog has primary hyperparathyroidism. I'd recommend chest radiographs and abdominal ultrasound to complete the workup, but they will likely be normal.
If you can do a parathyroid ultrasound, that would be recommended especially if surgical parathyroidectomy is planned.
Next question:
Sorry to be a bother, but no chance that this dog couldn't have hypercalcemia of malignancy?
How about renal renal secondary hyperparathyroidism or nutritional secondary hyperparathyroidism?
My Response:
Hypercalcemia of malignancy is possible but extremely unlikely in this dog. While hypercalcemia of malignancy does not always produce high levels of PTH-rp, the PTH concentration should be low in that situation, not high as is the case in your dog.
Unless the dog is eating some bizarre diet, we can rule out nutritional disease in a dog of this age, and I assume the dog is not in renal failure at this time, which largely eliminates that consideration. So primary hyperparathyroid disease remains the most likely by far.
Źródło: endocrinevet.blogspot.com
Treatment with arginine vasopressin (antidiuretic hormone of humans, dogs, and cats) or its analogues restores medullary hypertonicity and a normal urinary concentrating ability in animals with central diabetes insipidus. Historically, ADH tannate in oil, an extract of arginine vasopressin prepared from bovine and porcine pituitary glands, was administered every 2 to 3 days as needed to control polyuria and polydipsia. Because this product is no longer available, desmopressin acetate, a synthetic analogue of arginine vasopressin with prolonged and enhanced antidiuretic activity, has become the drug of choice for the treatment of central diabetes insipidus in dogs and cats.
DESMOPRESSIN PREPARATIONS
Desmopressin acetate is available in preparations for intranasal, parenteral (injectable), or oral administration (see Table below).
Nasal sprays or solutions of desmopressin
The nasal formulations are supplied with 2 different delivery systems: either a spray pump or a rhinal tube delivery system (see Table below), in which the desmopressin is “sprayed” or “blown” into the nose, respectively. Obviously, most dogs or cats will not tolerate either of these intranasal delivery methods. Drops placed in the conjunctival sac provide a more suitable alternative for animals.
With the rhinal tube delivery formulation (DDAVP Rhinal Tube®, Sanofi Aventis), the desmopressin is packaged with a small, calibrated plastic catheter so that exact amounts of the drug can be measured and administered. The calibrated rhinal tube has four graduation marks that measure amounts of 0.05 ml, 0.1 ml, 0.1 ml, and 0.2 ml and thereby can deliver doses of 5 to 20 µg of desmopressin). Although this system allows for accurate dosing, it is awkward to use. In addition, because this rhinal tube delivery system is not available as a generic product, this formulation is quite expensive.
 The most common intranasal formulations of desmopressin are marketed as nasal sprays or solutions equipped with compression pump that delivers 10 µg of drug with each spray. For use in dogs and cats, this spray bottle should be opened (a plier may be neccessary to break the seal) and the desmopressin solution transferred to a sterile vial; this dispensing vial then allows one to place the desmopressin drops within the animal’s conjunctival sac.
The most common intranasal formulations of desmopressin are marketed as nasal sprays or solutions equipped with compression pump that delivers 10 µg of drug with each spray. For use in dogs and cats, this spray bottle should be opened (a plier may be neccessary to break the seal) and the desmopressin solution transferred to a sterile vial; this dispensing vial then allows one to place the desmopressin drops within the animal’s conjunctival sac.
 These intranasal preparations of desmopressin are generally supplied as a concentration of 100 µg/ml; depending on the size of the drop, one drop of nasal solution corresponds to 1.5 to 4 µg of desmopressin. One highly concentrated nasal solution (1.5 mg/ml) is marketed for use in hemophilia (see Table below), but it should not be used to treat animals with diabetes insipidus because of the strong likelihood of overdosage.
These intranasal preparations of desmopressin are generally supplied as a concentration of 100 µg/ml; depending on the size of the drop, one drop of nasal solution corresponds to 1.5 to 4 µg of desmopressin. One highly concentrated nasal solution (1.5 mg/ml) is marketed for use in hemophilia (see Table below), but it should not be used to treat animals with diabetes insipidus because of the strong likelihood of overdosage.
In most cats and smaller dogs, 1 to 2 drops of the intranasal preparation administered once or twice daily are sufficient to control polyuria and polydipsia. Larger dogs may require up to 4 to 5 drops twice daily. Use of a tuberculin or insulin syringe allows for more accurate dosing. Application of desmopressin into the conjunctival sac may cause local irritation, as the solution is acidic. Some animals may object to the daily eye drops, making this route of administration ineffective.
Oral desmopressin tablets
The oral preparation of desmopressin is available both as a sublingual dissolve melt tablet (not suitable for treating cats) and as 0.1 mg and 0.2 mg tablets. Each 0.1 mg (100 µg) tablet is roughly comparable to 5-10 µg (1-2 large drops) of the nasal solution (see Table below).
 The tablet form of desmopressin is a more cost-prohibitive alternative compared with the conjunctival or subcutaneous routes of administration. The cost of daily oral desmopressin in animals is roughly 2.5-times that of the cost of conjunctival drops, and roughly 6 times the cost of subcutaneous injections of desmopressin. For some pet owners, however, the use of a tablet form may prove to be a more convenient, or the only possible route of administration that is possible.
The tablet form of desmopressin is a more cost-prohibitive alternative compared with the conjunctival or subcutaneous routes of administration. The cost of daily oral desmopressin in animals is roughly 2.5-times that of the cost of conjunctival drops, and roughly 6 times the cost of subcutaneous injections of desmopressin. For some pet owners, however, the use of a tablet form may prove to be a more convenient, or the only possible route of administration that is possible.
Injectable desmopressin solutions for SC or IV use
 An injectable sterile solution of desmopressin acetate (4 µg/ml) marketed for intravenous use is available (see Table) and can be used in animals with diabetes insipidus. However, the cost of the injectable desmopressin is approximately 7 to 15 times higher per µg than the intranasal preparation, making this formulation cost-prohibitive for use in most dogs and cats.
An injectable sterile solution of desmopressin acetate (4 µg/ml) marketed for intravenous use is available (see Table) and can be used in animals with diabetes insipidus. However, the cost of the injectable desmopressin is approximately 7 to 15 times higher per µg than the intranasal preparation, making this formulation cost-prohibitive for use in most dogs and cats.
To circumvent this cost iss
ue, the intranasal form of desmopressin – although not designed for parenteral use – can be given subcutaneously to cats with excellent results. Because the nasal forms of desmopressin are not considered to be sterile, however, it is best to first sterilize the product by passing the nasal solution through a 0.2 micron bacteriostatic syringe filter. Clinically the nasal and injectable preparations of desmopressin induce indistinguishable responses when administered subcutaneously.
To make dosing easier, the desmopressin is best administered with an U-100 low-dose insulin syringe. The solution can be diluted in sterile physiologic saline to make dosing easier.
The subcutaneous route of desmopressin administration has many advantages over the other routes of administration. These advantages include the following:
* First, drug appears to be most effective when administered via the subcutaneous route.
* Second, the duration of action is longer after subcutaneous injection than when administered orally or via the conjunctival sac.
* Third, because of the smaller subcutaneous doses required to control signs (about 15% and 40% of the oral and conjunctival doses, respectively), the cost of treatment is greatly reduced.
* Fourth, many cats seem to prefer long-term subcutaneous injections to the chronic use of eye drops or oral medication.

Company Web sites for more information:
- Sanofi Aventis
- Ferring Pharmaceuticals
- CSL Behring
- Bausch and Lomb
- Hospira
- Teva Pharmaceuticals
- Apotex Corporation
Desmopressin acetate is also available generically (many companies) and may also be known by the following synonyms and internationally registered trade names:
Concentraid®, D-Void®, Defirin®, Desmogalen®, Desmospray®, Desmotabs®, Emosint®, Minurin®, Nocutil®, Octim®, Octostim®, or Presinex® Źródło: endocrinevet.blogspot.com
Źródło: endocrinevet.blogspot.com
Once a diagnosis of diabetes insipidus has been confirmed, the next step to start replacement treatment with desmopressin.
Initial treatment with desmopressin
 Recommended initial doses of desmopressin vary depending on the route it is being administered. In most cats and smaller dogs, 1 to 2 drops of the intranasal preparation administered once or twice daily are sufficient to control polyuria and polydipsia (see Table below). Larger dogs may require up to 4 to 5 drops twice daily. Use of a tuberculin or insulin syringe allows for more accurate dosing. Application of desmopressin into the conjunctival sac may cause local irritation, as the solution is acidic. Some animals may object to the daily eye drops, making this route of administration ineffective.
Recommended initial doses of desmopressin vary depending on the route it is being administered. In most cats and smaller dogs, 1 to 2 drops of the intranasal preparation administered once or twice daily are sufficient to control polyuria and polydipsia (see Table below). Larger dogs may require up to 4 to 5 drops twice daily. Use of a tuberculin or insulin syringe allows for more accurate dosing. Application of desmopressin into the conjunctival sac may cause local irritation, as the solution is acidic. Some animals may object to the daily eye drops, making this route of administration ineffective.
With the subcutaneous route of administration, the initial recommended dose is 1.0 to 5.0 µg once or twice daily, depending on the size of the animals. If the nasal solution (100 µg /ml) were used for this purpose, one would inject only 0.01 to 0.05 ml (or 1 to 5 U with a U-100 insulin syringe). With the oral tablets, a starting dose of 0.05 mg to 0.2 mg (50 to 100 µg) once or twice daily is initiated.
Desmopressin dose adjustments
In dogs and cats with central diabetes insipidus, daily administration of desmopressin may completely eliminate polyuria and polydipsia. However, because of individual differences in absorption and metabolism, the dose required to achieve complete, around-the-clock control varies from patient to patient. The maximal effect of desmopressin occurs from 2 to 8 hours after administration, and the duration of action varies form 8 to 24 hours. Larger doses of the drug appear to both increase its antidiuretic effects and prolong its duration of action; however, expense can become a limiting factor for some owners.
No matter what route of administration is used, the daily dose should be gradually adjusted as needed to control signs of polydipsia and polyuria. The morning and evening doses can be adjusted separately if needed.
Adverse effects of desmopressin
Desmopressin is relatively safe for use in animals with central diabetes insipidus. Adverse effects of desmopressin are uncommon, but overdosage can lead to fluid retention, hyponatremia, and decreased plasma osmolality. Although extremely rare, fluid intoxication associated with desmopressin overdosage can lead to CNS disturbances including depression, increased salivation, vomiting, ataxia, muscle tremors, coma and convulsions. In such instances, furosemide can be given to induce diuresis.
To avoid the potential problem of overdosage, it is recommended that animals not be allowed free access to water immediately after each dose of desmopressin, especially if severe polydipsia and polyuria have redeveloped. Without such short-term (1 to 2 hours) water restriction, the cat many consume excessive amounts of water that cannot be subsequently excreted, as the desmopressin is absorbed and has its peak antidiuretic effects on the renal tubules.
Cost of desmopressin
The principle drawback with the use of any of the desmopressin preparations in the treatment of central diabetes insipidus is the drug’s considerable expense. The oral route of administration is the most expensive, while the subcutaneous route of administration (using the sterilized nasal solutions) is generally the most cost-effective.
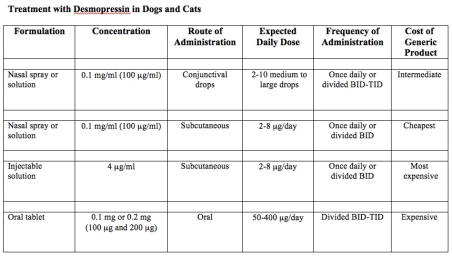
Źródło: endocrinevet.blogspot.com
I have a 12 year old male neutered Maine coon cat that has a free T4 (measured by equilibrium dialysis) of 57.9 nmol/L (normal is up to 50 nmol/L). The cat has stable weight and no real signs of hyperthyroidism. Other blood is good. Just wondering how we should follow up. I am contemplating re-assessing in a month or two? What is the correct approach to this case?
Thanks in advance.
My Response:
Monitor the cat's body weight, heart rate, thyroid size, and serum total T4 concentrations every 2-3 months.
Hyperthyroidism is a clinical diagnosis. The finding of a high serum free T4 value means nothing unless you have clinical signs, or a palpable thyroid nodule, or both.
Źródło: endocrinevet.blogspot.com
I don't know if I should even ask this but a situation in my family has me curious. My sister-in-law was diagnosed with pituitary-dependant Addison's disease over five years ago. She has been treated since that time with florinef and prednisone and has been doing well. Recently she had a recheck and an ACTH stimulation test while on treatment and she had a resting cortisol around 10 µg/dl and post around 20 µg/dl.
Now she's being weaned off of treatment and told her pituitary and adrenals are fine. I've never done an ACTH stimulation test on an animal I'm treating for Addison's disease after it's diagnosed and was wondering about the rational for doing that. Is human Addison's disease curable? Is there something completely different in human Addison's from the canine disease, or am I just missing something?
If anyone has an opinion and would like to share I'd appreciate it. Curiosity is getting the best of me. Thank you.
My Response:
It sounds to me as if they didn't believe the diagnosis of Addison's disease so they repeated the ACTH stimulation test. Addison's disease doesn't resolve once it develops (in either dogs or humans), and there is no such thing as "pituitary-dependent" Addison's disease.
On a brighter note, its's great news that your sister-in-law is normal!
Couldn't you theoretically get glucocorticoid deficiency from lack of production of ACTH? (Kind of like a central diabetes insipidus dog having a central lesion?)
My Response:
Absolutely. But pituitary ACTH deficiency is "secondary" hypoadrenocorticism, not Addison's disease.
Sorry to be such a stickler about terminology, but when Sir Thomas Addison described the disease that now uses his name, all of the patients had "primary" hypoadrenocorticism with complete destruction of both adrenal cortices.Therefore, the term Addison disease should be restricted to those patients with the primary form of the disorder (ie, those that have both glucocorticoid and mineralocorticoid deficiency).
There is no such disease as pituitary Addison's disease. Why? Because, like we all know, pituitary ACTH deficiency can not cause destruction of the adrenal gland or lead to mineralocorticoid deficiency. ACTH deficiency would only cause cortisol deficiency.
When we use the term Addison's so loosely, it becomes very confusing. It really would be best to avoid the use of the term completely. But we know that will never happen!
Źródło: endocrinevet.blogspot.com
This patient is a 5.5-year-old male neutered Westie that presents with the main complaints of polyuria and polydipsia (PU/PD). The results of a CBC and serum chemistry panal were normal. Strangely, however, the dog has glucosuria with no hyperglycemia. We have run multiple samples on different days of both blood and urine, all showing positive glucosuria but normoglycemia.
I submitted a urine cortisol:creatinine ratio (UCCR)to screen for Cushing's syndrome. It was not the ideal, home-caught, first in AM urine sample. It was free catch urine sample collected here at the clinic. Results show a UCCR ratio of 115. But the urine cortisol is 8.5 µg/dl (normal for Idexx laboratories reference range) and the creatinine is 23.1 mg/dl (low for the reference range). So, am I correct to interpret that the ratio is high because the creatinine is low and not because the cortisol is high? Can Cushing's syndrome be ruled out, or should I proceed to further Cushing's diagnostics?
My Response:
We do the UCC-ratio to correct for increased/decreased filtration and we therefore ought to look at the ratio, not the individual results. Therefore we cannot rule out Cushing's syndrome based on this result. You could, however, repeat measurements on a few home collected, morning samples. It is interesting that the serum alkaline phosphatase is normal in this, which, although still possible, makes hyperdrenocorticism less likely. It still is an important differential though. You could also run a fructosamine to check for chronic relative hyperglycemia.
Źródło: endocrinevet.blogspot.com









