This consult concerns a 7-year-old female spayed Pit-bull. Her last veterinary examination was over 5 years ago. She presented today with the main complain of chronic diarrhea of 3-weeks duration. On exam, the dog was very lethargic, weak, and 7-8% dehydrated.
On her in-house screening blood tests, primary abnormalities included severe hyponatremia (113 mEq/L; reference range, 139-154 mEq/L) and hyperkalemia (6.5 mEq/L; reference range, 3.5-5.5 mEq/L). The sodium:potassium ratio was low at 17.
On her fecal examination, I found a large number of whipworm eggs on a very poorly formed, mainly liquid sample, so I suspect she is loaded with these parasites.
Based on the finding of whipworms, I suspect that she does not have true hypoadrenocorticism (Addison’s disease) but rather pseudo-Addison’s disease. Do you agree? Do I need to do an ACTH stimulation test on this dog?
Do these dogs need IV fluids and other supportive treatment or is just treating them for the whipworms going to resolve the electrolyte disturbances without further treatment? Do we need mineralocorticoid replacement (e.g., Florinef or Percorten)?
My Response:
These are severe electrolyte derangements and definitely should be addressed as an emergency. Therefore, I would definitely hospitalize the dog and very slowly correct the low serum sodium concentrations. If hyponatremia is corrected too rapidly, central nervous system dysfunction and death can result.
During the chronic hyponatremia, the brain adapts to prevent cerebral edema. With rapid correction of serum sodium concentration, osmotic shifts and cerebral dehydration can occur. This may result in CNS changes including central pontine myelinosis. This can lead to severe neurological signs, including generalized weakness, ataxia, mental depression, and head pressing several days following correction of severe hyponatremia (1,2). Thus, we are faced with a conflict between the need to rapidly correct the dog’s hypovolemia while ensuring the serum sodium concentration does not increase too rapidly.
In dogs whose sodium depletion has been chronic or is severe (Na < 120 mEq/L), sodium replacement must be cautiously and slowly corrected, such that serum sodium rises by no more than 0.5 mEq/liter/hour or 15 mEq/liter in a 24 hour period (1,2). Overzealous and too rapid correction of serum sodium to normal levels must be avoided.
If serum sodium is < 120 mEq/L, it may be better to avoid normal saline because of the high sodium content (154 mEq/L), and instead administer either Lactated Ringer's (130 mEq/L sodium) or Normosol-R (140 mEq/L sodium). Although these two fluids also contain a small amount of potassium, intravascular volume correction will offset the potential risk of exacerbating hyperkalemia. Hypertonic saline administration (strengths of 513 or 858 mEq/L sodium) is contraindicated in initial treatment of severe hyponatremia.
As far as the definitive diagnosis, whipworm (Trichuris) infestation is a well-known cause of pseudo-Addison’s disease and those parasites should be treated (3). I would also do either an ACTH stimulation test or at least a resting serum cortisol concentration to rule out true Addison’s disease. If the basal cortisol is normal (>2.0 µg/dl) and/or the cortisol response to ACTH stimulation is normal, then we can be more certain that the serum electrolyte imbalances are due to the whipworms (3,4).
As long as fluid therapy is being administered, mineralocorticoid supplementation should not be necessary. Hopefully, the serum cortisol results will be back within the next day or so and then we can decide if other treatment for Addison’s disease is required.
Follow-up Report:
A basal serum cortisol concentration on this dog was normal at 3.3 µg/dl (reference range, 1-4 µg/dl). She responded very well to the initial fluid therapy and deworming, with normalization of the serum electrolytes and resolution of all of her diarrhea and other clinical signs.
The final diagnosis was indeed pseudo-Addison’s disease secondary to severe Trichuris (whipworm) infestation.
References:
- Brady CA, Vite CH, Drobatz KJ. Severe neurologic sequelae in a dog after treatment of hypoadrenal crisis. Journal of the American Veterinary - Medical Association1999;215:222-225, 210.
- MacMillan KL. Neurologic complications following treatment of canine hypoadrenocorticism. Canadian Veterinary Journal 2003;44:490-492.
- Graves TK, Schall WD, Refsal K, et al. Basal and ACTH-stimulated plasma aldosterone concentrations are normal or increased in dogs with trichuriasis-associated pseudohypoadrenocorticism. Journal of Veterinary Internal Medicine 1994;8:287-289.
- Lennon EM, Boyle TE, Hutchins RG, et al. Use of basal serum or plasma cortisol concentrations to rule out a diagnosis of hypoadrenocorticism in dogs: 123 cases (2000-2005). Journal of the American Veterinary Medical Association 2007;231:413-416.
Źródło: endocrinevet.blogspot.com
 Hyperthyroidism is a very common disorder of older cats. Also called thyrotoxicosis, hyperthyroidism is caused by an increase in production of the two main thyroid hormones — known as thyroxine (T4) and triiodothyronine (T3) from an enlarged thyroid tumor.
Hyperthyroidism is a very common disorder of older cats. Also called thyrotoxicosis, hyperthyroidism is caused by an increase in production of the two main thyroid hormones — known as thyroxine (T4) and triiodothyronine (T3) from an enlarged thyroid tumor.
Thyroid hormones affect nearly all the organs in the body; they play an important role in controlling the body's metabolic rate and thus the general activity level. This increased metabolic rate explains why hyperthyroid cats tend to burn up energy too rapidly and suffer weight loss despite having an increased appetite and food intake.
I first described the first cats with hyperthyroidism in 1979, only just over 30 years ago (1). Since then, hyperthyroidism has become the most common endocrine disorder in cats and a disease frequently diagnosed by all veterinarians in small animal practice. It is unclear why such a phenomenon has occurred.
Undoubtedly, increased awareness of hyperthyroidism by veterinary practitioners and by cat owners, easier availability of diagnostic tests, and a growing pet cat population have all played a role. However, its increased prevalence is not likely to be the result of an aging cat population alone. It may truly be a new disease that has just developed over the last three decades.
Incidence of feline hyperthyroidism varies around the world
The prevalence of hyperthyroidism varies geographically and at least anecdotally, regionally. It is a disease that is frequently diagnosed throughout North America, Europe, the United Kingdom, Australia, and New Zealand.
More recently, investigators in Japan have also reported an increasing number of cases. However, the disease appears to be much less common in other countries that have excellent veterinary care, such as in Spain and Hong Kong (2,3).
Thyroid gland changes associated with hyperthyroidism in cats
One of the largest endocrine gland in the body, the thyroid gland is found in the neck area, situated below the larynx (Adam's Apple) and adjacent to the trachea (windpipe).
In all species, including the cat, the thyroid gland is composed on 2 cone-like lobes or wings (in other words, there are 2 thyroid lobes that comprise the single thyroid gland). In most cats with hyperthyroidism, a benign or non-cancerous tumor (adenoma) develops in one or both of the thyroid lobes (4,5).
By contrast, thyroid cancer (carcinoma) is a rare cause of hyperthyroidism in cats, accounting for the tumors in less than 5% of hyperthyroid cats with hyperthyroidism (5,6).
But what’s causing these thyroid tumors to develop in the first place?
To date, the underlying factor(s) responsible for the thyroid changes remains obscure and is probably multifactorial. However, studies have indicated numerous environmental and nutritional risk factors, which may play a role in the why and how this disorder develops.
 Possible risk factors:
Possible risk factors:
- Diet composed entirely or primarily of canned cat food (7,9,15,18).
- Certain varieties of canned cat good, such as fish, liver, or giblet flavor (7, 10,18).
- Use of cat litter (7.9,18)
- Diets containing either excess or deficient amounts of iodine have been implicated (8, 17,19)
- Diets containing high amounts of selenium have also been suggested to have a role (11).
- Cans with plastic linings and pop-top lids may pose a greater risk than sachets or cans, which require a can opener to open them (13,18). This is potentially due to the release of chemicals such as bisphenol-A and bisphenol-F from the lacquer linings of the pop-top cans.
- Soy isoflavones (genistein and daidzein) are common constituents of commercially available cat foods and also may interfere with normal thyroid function (12,14).
- Regular use of insecticidal products (flea products) on the cat or fly sprays within the household (7,9,15).
- Exposure to herbicides and fertilizers (7,15).
- Exposure to flame-retardant chemicals contaminants including polybrominated diphenyl ethers (PBDEs). Excessive PBDEs have been identified in household dust from contaminated carpet padding, polyurethane foams, furniture and mattresses. High levels of PBDEs have both identified in the serum of both dogs and cats, indicating that this in a some environment contaminant (16,20).
So what’s the bottom line?
Given the variety of abnormalities and associations described it is likely that hyperthyroidism is a multifactorial disease. In other words, more than one factor is contributing to its development and pathogenesis.
It is also important to realize, however, that many of the risk factors listed above also exist in areas of the world where hyperthyroidism is considered relatively uncommon. This emphasizes the complexity of the problem in identifying the cause(s) of hyperthyroidism in cats.
References:
- Peterson ME, Johnson JG, Andrews LK. Spontaneous hyperthyroidism in the cat. Proceedings of the American College of Veterinary Internal Medicine 1979:p. 108.
- Wakeling J, Melian C, A. F, et al. Evidence for differing incidences of feline hyperthyroidism in London, UK and Spain. Proceedings of the 15th ECVIM-CA Congress 2005;2005.
- De Wet CS, Mooney CT, Thompson PN, et al. Prevalence of and risk factors for feline hyperthyroidism in Hong Kong. Journal of Feline Medicine and Surgery 2009;11:315-321.
- Gerber H, Peter H, Ferguson DC, et al. Etiopathology of feline toxic nodular goiter. Veterinary Clinics of North America Small Animal Practice 1994;24:541-565.
- Peterson ME, Ward CR. Etiopathologic findings of hyperthyroidism in cats. Veterinary Clinics of North America Small Animal Practice 2007;37:633-645, v.
- Hibbert A, Gruffydd-Jones T, Barrett EL, et al. Feline thyroid carcinoma: diagnosis and response to high-dose radioactive iodine treatment. Journal of Feline Medicine and Surgery 2009;11:116-124.
- Scarlett JM. Feline hyperthyroidism: A descriptive and case-control study. Preventive Veterinary Medicine 1988;6.
- Tarttelin MF, Ford HC. Dietary iodine level and thyroid function in the cat. Journal of Nutrition 1994;124:2577S-2578S.
- Kass PH, Peterson ME, Levy J, et al. Evaluation of environmental, nutritional, and host factors in cats with hyperthyroidism. Journal of Veterinary Internal Medicine 1999;13:323-329.
- Martin KM, Rossing MA, Ryland LM, et al. Evaluation of dietary and environmental risk factors for hyperthyroidism in cats. Journal of the American Veterinary Medical Association 2000;217:853-856.
- Foster DJ, Thoday KL, Arthur JR, et al. Selenium status of cats in four regions of the world and comparison with reported incidence of hyperthyroidism in cats in those regions. American Journal of Veterinary Research 2001;62:934-937.
- Court MH, Freeman LM. Identification and concentration of soy isoflavones in commercial cat foods. American Journal of Veterinary Research 2002;63:181-185.
- Edinboro CH, Scott-Moncrieff JC, Janovitz E, et al. Epidemiologic study of relationships between consumption of commercial canned food and risk of hyperthyroidism in cats. Journal of the American Veterinary Medical Association 2004;224:879-886.
- White HL, Freeman LM, Mahony O, et al. Effect of dietary soy on serum thyroid hormone concentrations in healthy adult cats. American Journal of Veterinary Research 2004;65:586-591.
- Olczak J, Jones BR, Pfeiffer DU, et al. Multivariate analysis of risk factors for feline hyperthyroidism in New Zealand. New Zealand Veterinary Journal 2005;53:53-58.
- Dye JA, Venier M, Zhu L, et al. Elevated PBDE levels in pet cats: sentinels for humans? Environmental Science and Technology 2007;41:6350-6356.
- Wakeling J, Elliott J, Petrie A, et al. Urinary iodide concentration in hyperthyroid cats. American Journal of Veterinary Research 2009;70:741-749.
- Wakeling J, Everard A, Brodbelt D, et al. Risk factors for feline hyperthyroidism in the UK. Journal of Small Animal Practice 2009;50:406-414.
- Edinboro CH, Scott-Moncrieff JC, Glickman LT. Feline hyperthyroidism: potential relationship with iodine supplement requirements of commercial cat foods. Journal of Feline Medicine Surgery 2010;12:672-679.
- Venier M, Hites RA. Flame retardants in the serum of pet dogs and in their food. Environmental Science and Technology 2011.
Źródło: endocrinevet.blogspot.com
 I have a 180 pound (82 kg) St. Bernard that was referred to me for supportive care overnight and to have an ACTH stimulation test done to rule out Addison's disease.
I have a 180 pound (82 kg) St. Bernard that was referred to me for supportive care overnight and to have an ACTH stimulation test done to rule out Addison's disease.
In the past, the maximal dose of synthetic cosyntropin (Cortrosyn) I've used in these large-breed dose is 250 µg per dog, or the whole vial of Cortrosyn. But there seems to be differing opinions on whether to give this dose or to use a 5-µg/kg dose calculated based upon the dog's body weight. Since this is such a large dog, should I administer 1 vial (250 µg), or should I give the 5 µg/kg-dose? In this dog, that would amount to giving 1.6 vials of the Cortrosyn!
Thanks so much for helping with my dilemma.
My Response:
The human dose for cosyntropin (Cortrosyn), no matter what the body weight, is a total dose of 0.25 mg (250 µg). So I would never administer more than 1 entire vial to any large dog, even if the calculated dosage turned out to be less than 5 µg/kg. For more information on the best protocol for ACTH stimulation testing in dogs and cats, see my previous blog post on the topic.
Studies (1,2) have reported that there is actually little difference in the serum cortisol responses in dogs when tested with the standard dose of Cortrosyn (5 µg/kg) and compared to a much lower dosage (0.5-1 µg/kg). So even with the low-dose protocol we now recommend 5 µg/kg), we already know that we are administering supra-physiological doses of ACTH to these dogs.
The bottom line about dosing with Cortrosyn for ACTH stimulation testing:
- The maximal dose of Cortrosyn for any sized dog is a total dose of 250 µg.
- Even if a dog gets a bit less than the calculated 5 µg/kg-dose (no matter what the body weight), this will still produce a maximal serum cortisol response and the ACTH stimulation test results will still be completely valid.
References:
- Kerl ME, Peterson ME, Wallace MS, Melián C, Kemppainen RJ. Evaluation of a low-dose synthetic adrenocorticotropic hormone stimulation test in clinically normal dogs and dogs with naturally developing hyperadrenocorticism. J Am Vet Med Assoc 1999,214:1497-501.
- Martin LG, Behrend EN, Mealey KL, Carpenter DM, Hickey KC. Effect of low doses of cosyntropin on serum cortisol concentrations in clinically normal dogs. Am J Vet Res 2007;68:555-60.
Źródło: endocrinevet.blogspot.com
 Addison’s disease (also referred to as hypoadrenocorticism or adrenal insufficiency) is an uncommon disorder in which the adrenal cortex fails to secrete sufficient amounts of its steroid hormones. These include cortisol, aldosterone, or both hormones (see my last post on Addison's disease for more information). Because these adrenal hormones are essential for life, the consequences can be life threatening when they are not secreted in normal amounts.
Addison’s disease (also referred to as hypoadrenocorticism or adrenal insufficiency) is an uncommon disorder in which the adrenal cortex fails to secrete sufficient amounts of its steroid hormones. These include cortisol, aldosterone, or both hormones (see my last post on Addison's disease for more information). Because these adrenal hormones are essential for life, the consequences can be life threatening when they are not secreted in normal amounts.
Natural course of hypoadrenocorticism
During the initial stages of this Addison’s disease, the clinical signs are generally vague and nonspecific, making diagnosis very difficult. As time goes on, more and more of the adrenal cortex is destroyed in these dogs.
Approximately 90% of the adrenal cortex must be destroyed before
overt clinical signs of Addison’s disease are generally observed.
Without early diagnosis and treatment, the disease can result in a phenomenon known as an Addisonian crisis. The dog collapses in shock due to its inability to adapt to their circulatory requirements in time stress. Blood sugar may drop dangerously low. Potassium levels soar and disrupt the heart rhythm because there is not enough conserved sodium to exchange for potassium. Heart rate slows, and arrhythmias may result. The dog may not survive this episode.
Approximately 30% of dogs with Addison's disease are NOT diagnosed
until they develop a life-threatening adrenal crisis.
Making the diagnosis
Because of the nonspecific and vague clinical signs that are seen in dogs with Addison's disease, this disorder has earned a distinct medical nickname —The Great Imitator.
The first step in making a diagnosis of Addison’s disease is to perform basic blood tests, which commonly show abnormalities that point to hypoadrenocorticism as the potential problem. For example, a complete blood count (CBC) may reveal the following changes that could indicate Addison’s disease:
- Anemia (low red blood cell count)
- High numbers of lymphocytes, a type of white blood cell
- High numbers of eosinophils, another type of white blood cell
A serum chemistry profile generally reveals one or more of the following abnormalities in dogs with Addison’s disease:
- High potassium concentration
- Low sodium concentration
- Low sodium/potassium ratio (Na:K less than 27:1)
- High urea nitrogen concentration
- High creatinine concentration
- High phosphorus concentration
- High calcium concentration
- Low glucose concentration
Chest and abdominal x-rays may reveal a smaller than normal heart and liver. These radiology changes are the result of the Addisonian dog’s shock-like state, which reduces the circulating fluid volume in the body.
Confirming the diagnosis
When screening laboratory tests are consistent with Addison’s disease, the next step is to directly evaluate the dog’s adrenal function. To do this, the veterinarian will perform an adrenocorticotropic hormone (ACTH) stimulation test. This the only definitive test for Addison's disease and allows us to confirm the diagnosis.
To perform an ACTH stimulation test in a dog with suspected Addison’s disease, we collect a blood sample to measure the level of cortisol. We then administer a dose of ACTH, the pituitary hormone responsible for the release of corticosteroids in times of stress. After an hour, we collect a second blood sample to measure the dog’s serum cortisol concentration once again. (For more information on the protocol for ACTH stimulation testing, see my blog post written for vets and vet technicians).
In healthy dogs, the baseline cortisol concentration is normal, and ACTH will stimulate the adrenal gland to secrete cortisol. Generally, a 3 to 5 fold increase in cortisol occurs after ACTH injection (see Figure below).
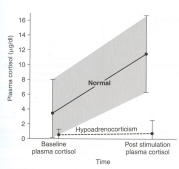
In contrast, dogs with Addison’s disease (hypoadrenocorticism) have a low baseline cortisol concentration (before we administer the ACTH) and show little or no rise in the cortisol value after the ACTH injection (see Figure above). The subnormal levels of cortisol combined with the lack of cortisol response after ACTH stimulation is diagnostic for Addison's disease.
Differentiating primary hypoadrenocorticism from secondary and atypical hypoadrenocorticism
The finding of severe serum electrolyte disturbances (i.e, high potassium, low sodium, low sodium/potassium ratio), together with subnormal cortisol values that fails to rise after ACTH injection (see Figure above) is diagnostic for primary Addison's disease.
However, in dogs with secondary and atypical hypoadrenocorticism, the serum electrolytes are generally normal, making diagnosis more difficult. In these dogs, further diagnostic testing is recommended, including determination of the pituitary hormone, ACTH, or measuring serum concentrations of aldosterone - the adrenal hormone responsible for maintaining normal serum electrolytes. For more information, see this blog post on atypical Addison's disease I wrote for veterinarians.
In my next post, I'll discuss our treatment options for this life-threatening disease.
Źródło: animalendocrine.blogspot.com
Addison's disease, the common name for hypoadrenocorticism or adrenal insufficiency, as I have discussed in my last two blog posts, is a disease with vague clinical features that are common in many other ailments, making diagnosis difficult in many cases. But once Addison's disease is correctly diagnosed, a properly treated dog can live a normal and happy active life.
 The missing adrenal hormones
The missing adrenal hormones
The adrenal, one on each kidney, is made up of two layers, the cortex and the medulla. The inner medulla secretes epinephrine (adrenaline) and is not affected by Addison's disease. The outer cortex layer secretes two corticosteroid hormones, cortisol and aldosterone, both of which are deficient in Addison's disease.
Aldosterone is a corticosteroid hormone (more specifically, a mineralocorticoid — think minerals: salt, sodium, potassium) responsible for maintaining normal circulating electrolyte levels. Once secreted, aldosterone acts on the kidney to conserve sodium, excrete potassium, and retain needed water.
Cortisol is also a corticosteroid hormone (in this case, a glucocorticoid — think glucose, sugar, energy) that is essential for life. It supports a variety of important cardiovascular, metabolic, immunologic, and stress functions.
Not all forms of hypoadrenocorticism are treated the same
There are three forms of hypoadrenocorticism: primary, secondary and atypical Addison's disease.
- Primary Addison's disease is most commonly is the result of immune-mediated damage to the glands.
- Atypical Addison's disease is a poorly understood disorder, thought to generally be an early stage of primary Addison's disease.
- Secondary hypoadrenocorticism results from a deficiency of the pituitary hormone, adrenocorticotropic hormone (ACTH). Without circulating ACTH, cortisol cannot be secreted by the adrenal glands.
It is important which form of hypoadrenocorticism is present in order to provide the correct treatment. In primary hypoadrenocorticism, both cortisol and aldosterone are deficiency and must be replaced for life. In atypical and secondary hypoadrenocorticism, on the other hand, only the glucocorticoids need to be replaced, at least initially.
Treating the acute adrenal crisis: A true medical emergency
Untreated, hypoadrenocorticism (especially primary Addison's disease) can lead to an adrenal crisis. An adrenal crisis is a medical emergency that requires intravenous fluids and glucocorticoids to restore the body’s levels of fluids, salt, and sugar to normal.
Once stabilized, the dog can then be treated with glucocorticoid and mineralocorticoid replacement therapy at home.
Treating chronic hypoadrenocorticism: A lifelong disease
Depending of the subtype of hypoadrenocorticism, synthetic corticosteroid drugs that act like mineralocorticoid or glucocorticoids used for hormone replacement therapy.
Mineralocorticoid treatment
For mineralocorticoid (aldosterone) replacement, either an oral medication called fludrocortisone acetate (Florinef™) or the injectable desoxycorticosterone pivalate (DOCP; Percorten-V™) is used.
 We typically institute treatment with DOCP (Percorten-V) at a dosage of 2.2 mg/kg, subcutaneously or intramuscularly, every 25 to 30 days. Side effects associated with DOCP therapy are rare. This dosage interval is effective in almost all dogs, and most are well controlled with a DOCP injection every 4 weeks.
We typically institute treatment with DOCP (Percorten-V) at a dosage of 2.2 mg/kg, subcutaneously or intramuscularly, every 25 to 30 days. Side effects associated with DOCP therapy are rare. This dosage interval is effective in almost all dogs, and most are well controlled with a DOCP injection every 4 weeks.
Initially, serum kidney and electrolyte concentrations should be monitored at approximately 2-weeks intervals in order to determine the drug’s peak effect and to help make necessary dosage adjustments. Once stabilized, serum electrolyte and creatinine concentrations are checked every 3 to 6 months. Because DOCP is a pure mineralocorticoid and has no glucocorticoid activity, it is essential that dogs receive concurrent glucocorticoid supplementation (see below).
 Fludrocortisone is a synthetic corticosteroid that possesses moderate glucocorticoid activity as well as having marked mineralocorticoid potency. By comparison, fludrocortisone has 10 times the glucocorticoid activity and 125 times the mineralocorticoid activity of cortisol. In this regard, fludrocortisone is very different than DOCP, which possess no glucocorticoid activity.
Fludrocortisone is a synthetic corticosteroid that possesses moderate glucocorticoid activity as well as having marked mineralocorticoid potency. By comparison, fludrocortisone has 10 times the glucocorticoid activity and 125 times the mineralocorticoid activity of cortisol. In this regard, fludrocortisone is very different than DOCP, which possess no glucocorticoid activity.
If fludrocortisone acetate is employed as mineralocorticoid supplementation, we recommend an initial oral dosage of approximately 0.02 mg/kg/day.
After initiation of fludrocortisone therapy, serum electrolyte and creatinine concentration should be monitored weekly, with the dosage adjusted by 0.05-0.1 mg/day increments until values have stabilized within the reference range. Once this is achieved, the dogs should be reevaluated monthly for the first 3 to 6 months of therapy, then every 3 to 6 months thereafter.
For dogs that have atypical or secondary Addison’s, mineralocorticoid replacement therapy with DOCP or fludrocortisone aren't needed because the production of aldosterone isn’t effected and the serum electrolytes remain in balance.
Glucocorticoid treatment
In addition to replacing deficient mineralocorticoids in dogs with Addison's disease, the missing glucocorticoids must also be replaced. This is typically done with an oral form of the synthetic glucocorticoids prednisone, prednisolone, or hydrocortisone. With atypical Addison's and secondary forms of hypoadrenocorticism, glucocorticoid replacement is all that is needed, at least initially, since these dogs do not have serum electrolyte abnormalities.
 |
 |
 |
The correct dose of the supplemented glucocorticoids, such as prednisone, cannot be measured with a blood test. Rather it's determined by your observations: the lowest dose that keeps your dog symptom free, happy and eating! The most common side effect of overdosage is an increase in thirst and urination, which can be intense in some dogs.
Prognosis of Addison's disease
With proper treatment, the long-term prognosis is excellent. While your dog with Addison’s disease will need medications and monitoring for the rest of his life, most dogs with Addison’s can return to their favorite activities. You will help your dog lead a normal, active and fun-filled life.
Źródło: animalendocrine.blogspot.com
I n the past four years, diabetes rates among dogs in the U.S. increased roughly 33% among dogs and 16% among the nation's cat population, per a national analysis of pet health.
n the past four years, diabetes rates among dogs in the U.S. increased roughly 33% among dogs and 16% among the nation's cat population, per a national analysis of pet health.
According to the report, other common health problems identified among pets include flea and tick infestations, internal parasites, and outer ear inflammations.
See the complete article published today in The Record (Hackensack, New Jersey)
Źródło: animalendocrine.blogspot.com









